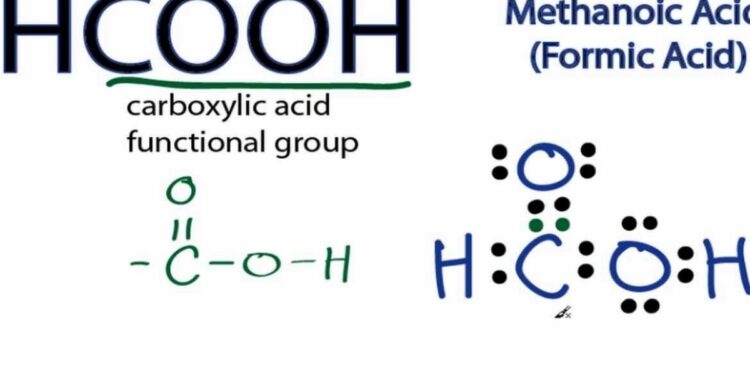
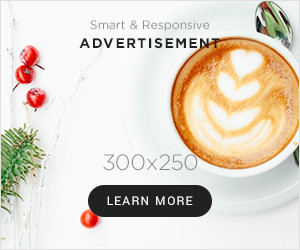
The study of formic acid, commonly represented by HCOOH, has long been a cornerstone of organic chemistry. hcooh structure One of the most important aspects in understanding this molecule is analyzing the structure for hcooh. The molecular structure of HCOOH determines its physical and chemical properties, which in turn influence its reactivity, acidity, and role in various industrial and biological applications. Researchers have extensively studied the structure for hcooh to gain insights into its behavior in aqueous solutions, its ability to donate protons, and its interaction with other chemical compounds. Understanding the structure is crucial for predicting chemical reactions, designing synthetic pathways, and exploring its practical applications in different fields.
Molecular Composition and Geometry
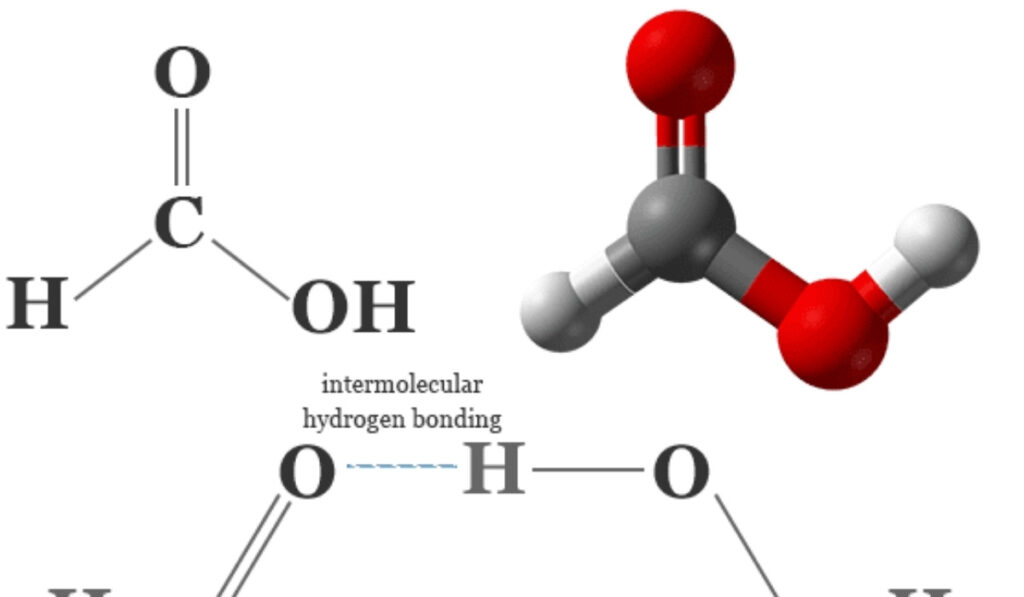
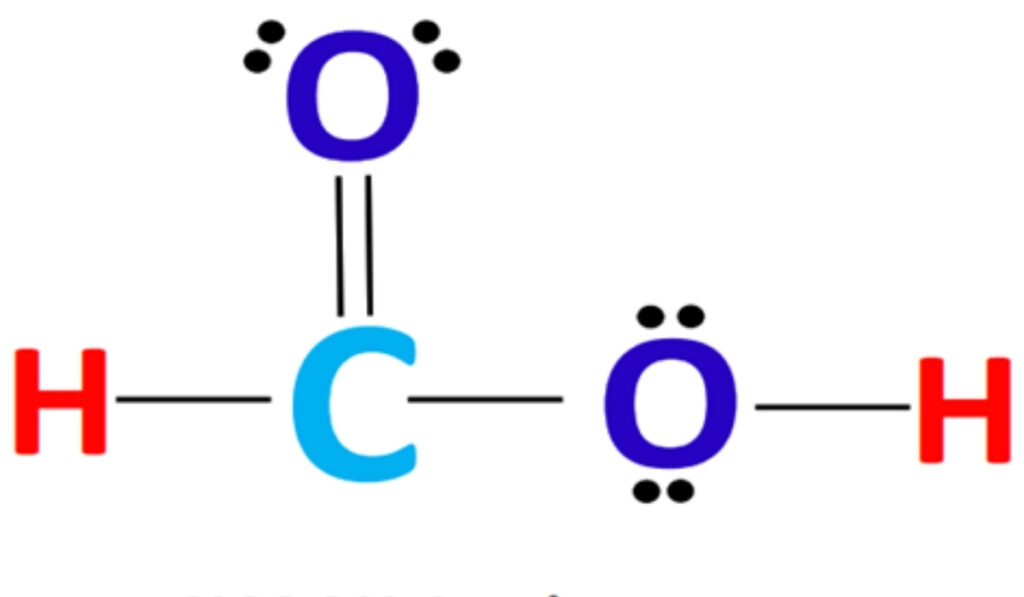
The structure for hcooh consists of a single carbon atom bonded to a hydroxyl group and a formyl group, forming a planar molecule with sp2 hybridization at the carbon center. The geometry around the carbon atom is trigonal planar, with bond angles approximating 120 degrees. This arrangement allows for resonance stabilization between the carbonyl and hydroxyl groups, contributing to the molecule’s acidity and reactivity. Chemists studying the structure for hcooh emphasize that this geometry facilitates hydrogen bonding, both intermolecularly and with solvents, which is a key factor in its solubility and interaction with other chemicals.
Bonding and Resonance in HCOOH
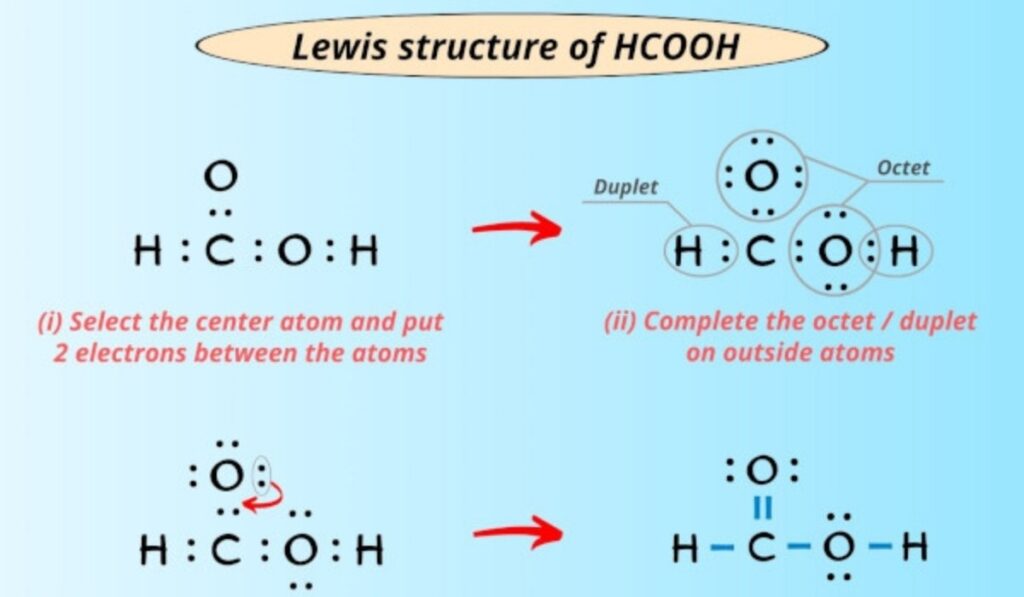
The structure for hcooh exhibits significant resonance, where the electrons in the carbonyl and hydroxyl groups can delocalize across the molecule. This resonance is essential in understanding the molecule’s chemical stability and its acidic properties. The delocalization of electrons reduces the overall energy of the molecule, making it less reactive in certain contexts while enhancing its ability to participate in specific reactions, such as esterification and oxidation. By studying the structure for hcooh, chemists can predict how it will behave under various chemical conditions and how it can be effectively used in industrial processes.
Physical Properties Influenced by Structure
The physical properties of HCOOH, including its boiling point, melting point, and solubility, are directly influenced by the structure for hcooh. Hydrogen bonding is a primary contributor to its high boiling point relative to its molecular weight. The ability of HCOOH to form dimers in the liquid phase is another characteristic resulting from its structural arrangement. Understanding the structure for hcooh allows chemists and engineers to manipulate its physical properties for specific applications, such as in solvents, preservatives, or as a reagent in chemical syntheses.
Acidity and Chemical Behavior
Formic acid is a relatively strong carboxylic acid due to its structural characteristics. The electron-withdrawing effect of the carbonyl group stabilizes the conjugate base, enhancing acidity. The structure for hcooh allows for easy proton donation, which makes it reactive in acid-catalyzed reactions and suitable for use in various industrial processes, such as leather tanning and textile processing. By examining the structure for hcooh, chemists can understand the molecule’s behavior in aqueous solutions and its interaction with bases, which is essential for both laboratory and industrial applications.
Industrial Applications and Relevance
The structure for hcooh underpins many of its industrial applications. It is widely used as a preservative in food and feed, as a reducing agent in chemical syntheses, and as an intermediate in the production of formate salts. Its ability to participate in redox reactions, facilitated by its molecular structure, makes it invaluable in several manufacturing processes. Chemical engineers study the structure for hcooh to optimize reaction conditions, improve yields, and ensure safety in industrial environments where formic acid is employed on a large scale.
Biological Significance
The structure for hcooh is also relevant in biological systems. Formic acid is naturally produced by some insects, such as ants, as a defense mechanism. Its ability to penetrate biological membranes and its reactivity are directly related to the structural arrangement of its functional groups. Biochemists and pharmacologists analyze the structure for hcooh to understand its effects on living organisms, its metabolic pathways, and its potential applications in medicine and biotechnology. The molecular geometry allows for interactions with enzymes and cellular components, explaining its biological potency.
Solvent Interactions and Hydrogen Bonding
Hydrogen bonding is a central feature of the structure for hcooh, influencing its interactions with solvents and other molecules. Its ability to act as both a hydrogen donor and acceptor allows it to form strong associations in aqueous and polar organic solvents. These interactions are crucial in processes such as extraction, crystallization, and chemical reactions. By studying the structure for hcooh, chemists can design more efficient reaction systems, predict solubility trends, and optimize solvent choices for industrial and laboratory applications.
Environmental and Safety Considerations
Understanding the structure for hcooh is important for assessing its environmental and safety implications. The molecule’s reactivity, volatility, and ability to participate in chemical reactions influence its handling, storage, and disposal. Environmental chemists and safety officers use knowledge of the structure for hcooh to develop protocols for safe industrial usage, spill management, and waste treatment. Proper understanding of the molecular properties ensures that formic acid can be used effectively without posing significant risks to humans or the environment.
Future Research and Technological Applications
The structure for hcooh continues to be a subject of research for chemists seeking to explore new applications. Advances in computational chemistry and spectroscopy have allowed for a deeper understanding of electron distribution, reactivity, and molecular interactions. These insights pave the way for novel applications in catalysis, renewable energy, and material science. Researchers analyzing the structure for hcooh aim to leverage its unique properties to develop environmentally friendly processes, improve industrial efficiency, and discover innovative uses in chemistry and biology.
Conclusion
The study of HCOOH, particularly the structure for hcooh, reveals the intricate relationship between molecular geometry, chemical behavior, and practical applications. From industrial processes to biological interactions, the structural arrangement of HCOOH determines its functionality, stability, and reactivity. Understanding the structure for hcooh is essential for chemists, engineers, and researchers working with this versatile molecule, enabling optimized use in chemical synthesis, environmental management, and innovative scientific applications. The insights gained from structural analysis provide a foundation for continued exploration of formic acid in both academic and industrial settings.
Frequently Asked Questions
1. What is the structure for hcooh?
- It consists of a carbon atom bonded to a hydroxyl group and a formyl group in a planar arrangement.
2. How does the structure for hcooh affect acidity?
- The carbonyl group stabilizes the conjugate base, enhancing its acidic properties.
3. Can the structure for hcooh form hydrogen bonds?
- Yes, it forms strong hydrogen bonds with solvents and other molecules.
4. What are the main industrial uses of HCOOH?
- It is used in preservatives, chemical synthesis, and production of formate salts.
5. Why is understanding the structure for hcooh important in research?
- It helps predict reactivity, optimize industrial processes, and explore new applications.